Research Interests:
Neuron-glial cell interactions
Glial cells once were relegated to the status of supporting cells for neurons. Recent research, however, has cast the glia in a new light, revealing them to be involved with neurons in complex interactions that strongly affect the viability, growth, and functioning of the neurons. During development, glial cells have especially important roles in the survival and migration of neurons, guidance of axons, and formation of neuronal branching patterns. But most interestingly, our work and that of other labs has shown that the neurons in turn influence glial cell development, suggesting that a dynamic interaction between the two cell classes is a necessary element in correct development of the nervous system. Because recognition that these neuron-glial cell interactions can have such profound effects is so recent, our knowledge of the repertoire of interactions undoubtedly is incomplete and our understanding of the underlying mechanisms is still nascent. Similarly, though it is widely recognized that glial cells and neurons interact in the more mature brain to modulate neuronal function, the mechanisms remain poorly understood.
To study the role of neuron-glia interactions during development and in the functioning CNS, we use both the olfactory system of the moth Manduca sexta and the olfactory and larval motor systems of Drosophila.
Manduca studies on development of the olfactory pathway
The moth is an animal whose sensory periphery is easily accessible and readily manipulated experimentally, and whose olfactory pathway develops during metamorphosis. [Fig 1] Its mature circuitry and olfactory behavior have been extensively studied, especially in the Hildebrand lab, providing us with a robust foundation for our developmental studies.
Figure 1
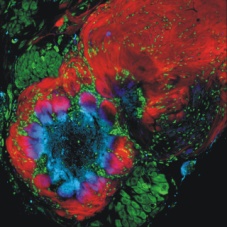 |
The olfactory (antennal) lobe of the male moth Manduca sexta. Red, olfactory sensory axons traveling in the nerve (upper right), in the nerve layer of the lobe, and entering into the ovoid glomerular structures where they synapse with intrinsic neurons of the antennal lobe. Green, cell bodies of neurons, which appear in two clusters, and glial cells, which are distributed around each glomerulus and within the nerve layer and antennal nerve. Blue, dendrites of antennal lobe neurons. Pink, overlap of sensory terminals and dendrites of antennal lobe neurons. |
During the period, olfactory receptor neurons send their axons to the antennal (olfactory) lobe. There the axons induce the formation of structures called glomeruli, which appear in virtually all olfactory systems across the animal kingdom. We have found that glial cells have a vital role in newly forming glomeruli. When the glial cell population is severely reduced, the mature antennal lobe lacks glomeruli. The involvement of glial cells in the development of the antennal lobe begins even earlier, however, when the receptor axons first approach the antennal lobe. Just outside the lobe, the axons enter a glia-rich domain where they undergo a sorting process that changes their topographic organization to a chemotopic organization, a process that also must be accomplished by vertebrate olfactory axons. In the moth, the axons fail to sort properly in glia-deficient olfactory systems, strongly suggesting that a glia-neuron interaction underlies the process of axon sorting.
In ongoing studies done in collaboration with Leslie Tolbert and Nick Gibson, we are using a variety of techniques to examine the cellular and molecular bases of the neuron-glia interactions in the sorting zone region of the nerve, where olfactory sensory axons are sorted into region-specific or glomerulus-specific bundles, and in the developing glomeruli. These include cell culture, electrophysiology, immunocytochemistry, electron microscopy confocal microscopy and live-cell imaging. [Fig. 2]
Figure 2
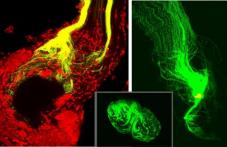 |
The axonal sorting zone, a discrete, glia-rich region of the antennal nerve at its entry into the antennal lobe. DiI placed distally reveals changes in axon trajectory and dispersal of neighboring axons in the nerve to targets throughout the antennal lobe. DiO placed in the sorting zone close to the lobe reveals that axons from across the nerve leave the sorting zoe destined for one or a small number of glomeruli. |
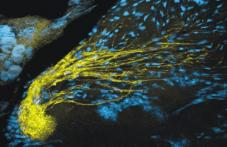 |
Olfactory sensory axons gathered in the sorting zone and terminating in a single glomerulus. |
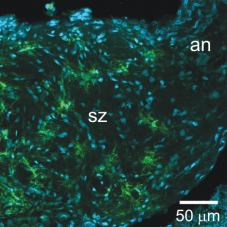 |
Glial cells in the sorting zone expressing a GABA transporter. |
These studies have revealed roles for both the EGF receptor and the FGF receptor in axon ingrowth, axonal fasciculation, and glial migration, and also provided evidence suggesting an early role for GABA in dendritic branching mediated by glial responses to GABA. [Fig 3]
Figure 3
 |
GABAergic antennal lobe interneuron in culture. |
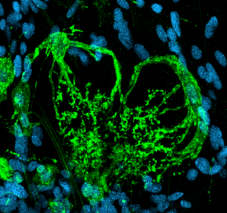 |
Complex glial cells whose branches extend to the base of a glomerulus. Cell expresses a GABA transporter. |
Also, serial block face imaging and reconstruction of part of the glial network in the sorting zone by Amanda Levy, an undergraduate in the lab, has revealed the enormous extent of the glial network, even at early developmental stages. [Fig 4]
Figure 4
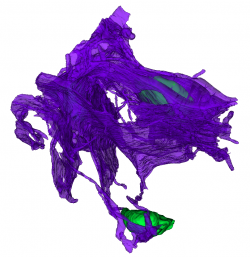 |
Partial 3D reconstruction of the glial network in the sorting zone region of the olfactory nerve from serial block face electron micrographs. Green, glial cell nuclei. Magenta, extensive sheets of glial processes surrounding bundles of axons of the olfactory sensory receptors. These are not shown. |
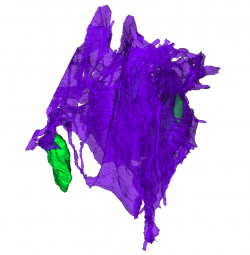 |
Given the many organizational and molecular similarities between the moth and the vertebrate olfactory systems, we expect that insights gained in studying the moth system will be broadly applicable in understanding the role of neuron-glia interactions in the developing vertebrate olfactory system.
Interaction between olfactory axons and peripheral glia during development
In Manduca, the organization of the olfactory nerve closely resembles that in the mammalian olfactory nerve. Glial cells form a network of processes that ensheathe bundles of 60-100 small-diameter axons, as a group. To date, an undergraduate student in the lab, Mounir Koussa (now a graduate student at Harvard Neurobiology), detailed the development of the glial network during the period of olfactory axon ingrowth. The peripheral glial cells arise in the antennal olfactory epithelium and migrate into the nerve just after the outgrowing sensory axons. The glial cells migrate in chains and some divide during the migratory period. Whole-cell and perforated-patch recordings showed at least 2 potassium currents and an R-like calcium current. Blocking spontaneous activity in the axons with TTX in vivo resulted in reduction of the glial calcium current. The glial cells are highly dye-coupled initially, but de-couple as they begin to extend the processes that eventually will form the glial network. Migration can be blocked in vivo either by blocking the R-like current or by blocking the GABAA receptor with picrotoxin. The accessibility of the nerve and its length (2 mm intracranially), as well as our ability to eliminate the glial network by blocking migration, allows us to interrogate the physiological interaction between active axons and the glial cells when the nerve is activated by behaviorally relevant olfactory stimuli.
Neuron-glia interactions in the motor neuropil of the ventral ganglion in Drosophila
In Drosophila, we are focusing on neuron-glia interactions in the larval ventral ganglion, where previous studies of the morphology and physiological behavior of the motoneurons completed in other labs have set a strong foundation for our studies of the role of glial cells in modulating motoneuron activity. A graduate student in the lab, Sarah MacNamee, is leading a study in which she is using current-clamp, whole-cell recording, and calcium imaging techniques to record from neuropil-associated glial cells to examine the reciprocal conversation between the motoneurons and the glial cells. Two undergraduate students on her team, Si Woo Lee and Nancy Anaya, are studying the anatomical relationships among glial processes and motor neurons [Fig 5, SWL pics] and the consequences of deleting these glial cells via neuropil-glia-targeted temperature-sensitive activation of apoptosis on patterns of locomotion.
Selected Publications:
Gibson NJ, Tolbert LP, Oland LA (2012) Activation of glial FGFRs is essential in bidirectional axon-glia signaling during development of the moth olfactory system. PLoS One 7: e33828.
Mallory HS, Gibson NJ, Hayashi JH, Nighorn AJ, Oland LA (2012) Direct and glia-mediated effectos of GABA on development of central olfactory neurons. Neuron Glia Biology July 2012. doi:10.1017/S1740925X12000075
Oland LA, Tolbert LP. (2011) Roles of glial cells in neural circuit formation: Insights from insects. Glia 59:1273-1295.
Koussa MA, Tolbert LP, Oland LA. Development of a glial network in the olfactory nerve: role of calcium and neuronal activity. Neuron Glia Biology Sep 21:1-17. doi:10.1017/S1740925X11000081
Oland LA, Gibson NJ, Tolbert LP. 2010, Localization of a GABA transporter to glial cells in the developing and adult olfactory pathway of the moth Manduca sexta. J Comp Neurol. 518(6):815-838.
Gibson NJ, Tolbert LP, Oland LA. 2009, Roles of specific membrane lipid domains in EGF receptor activation and cell adhesion molecule stabilization in a developing olfactory system, PLoS One, 4(9):e7222.
Oland LA, Biebelhausen JP, Tolbert LP. 2008, Glial investment of the adult and developing antennal lobe of Drosophila, J Comp Neurol. 509(5):526-550.
Heil JE, Oland LA, Lohr C. 2007, Acetylcholine-mediated axon-glia signaling in the developing insect olfactory system, Eur J Neurosci. 26(5):1227-1241.
Tolbert LP, Oland LA, Tucker ES, Gibson NJ, Higgins MR, Lipscomb BW. 2004, Bidirectional influences between neurons and glial cells in the developing olfactory system, Prog Neurobiol. 73(2):73-105.
Abeytunga DT, Glick JJ, Gibson NJ, Oland LA, Somogyi A, Wysocki VH, Polt R. 2004. Presence of unsaturated sphingomyelins and changes in their composition during the life cycle of the moth Manduca sexta. J Lipid Res, 45:1221-31
Tucker ES, Oland LA, Tolbert LP. 2004. In vitro analyses of interactions between olfactory receptor growth cones and glial cells that mediate axon sorting and glomerulus formation. J Comp Neurol, 472:478-95
Slavish JP, Friel DK, Oland LA, Polt R. 2004. New PDMP analogues inhibit process outgrowth in an insect cell line. Bioorg Med Chem Lett., 14(6):1487-90.
Oland LA, Pott WM, Howard CT, Inlow M, Buckingham J. 2003. A diffusible signal attracts olfactory sensory axons toward their target in the developing brain of the moth. J Neurobiol, 56:24-40
Oland LA, Evans S. 2000, The tracheal system of the developing primary olfactory pathway of Manduca sexta: tracheae do not play a guidance or targeting role for ingrowing receptor axons, Arthropod Struct Dev. 29(3):185-196.

